

They occur between polar, covalently bound atoms in different molecules. They are also called inter-molecular forces.

Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. Hydrogen bonds are also responsible for zipping together the DNA double helix. Individual hydrogen bonds are weak and easily broken however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. This type of bond is common and occurs regularly between water molecules. This interaction is called a hydrogen bond.
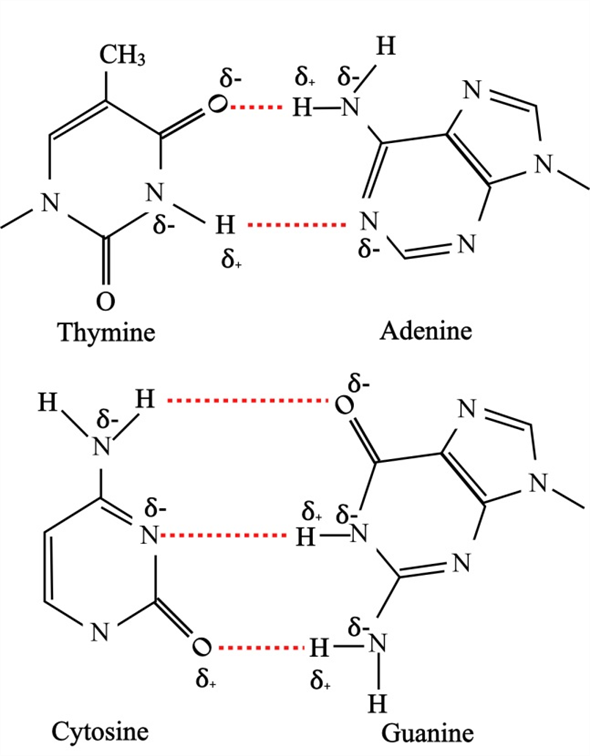
When this happens, a weak interaction occurs between the δ+ of the hydrogen from one molecule and the δ– charge on the more electronegative atoms of another molecule, usually oxygen or nitrogen, or within the same molecule. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen’s electron is pulled more strongly toward the other element and away from the hydrogen. Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and DNA, the building block of cells. Without these two types of bonds, life as we know it would not exist. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Weaker bonds can also form between molecules. However, not all bonds are ionic or covalent bonds. Ionic bonds are not as strong as covalent, which determines their behavior in biological systems. Ionic and covalent bonds between elements require energy to break. Model a hydrogen bond and identify its unique qualities.


 0 kommentar(er)
0 kommentar(er)
